In-vitro in-vivo extrapolation (IVIVE)
The in-vitro in-vivo extrapolation method implemented in MCRA is based on the following prerequisites:
For one substance, the index substance, a reliable point of departure is available for the adverse outcome of interest obtained from an in-vivo assay (i.e., external dose).
There are other substances for which there is a dose-response model available from an in-vitro assay on a response representing an early key event of the adverse outcome for these substances and the index substance.
In IVIVE, these RPFs, in combination with the known hazard characterisation of the index substance, can be used to derive hazard characterisations for the other substances as well. Figure 78 shows the conceptual model that forms the basis of the IVIVE methodology of MCRA.
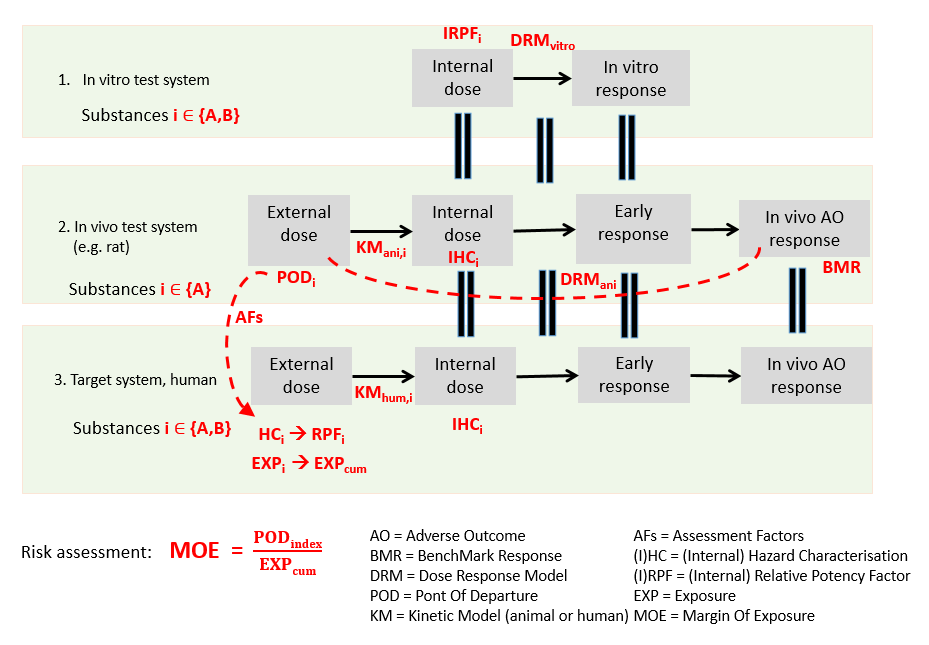
Figure 78 Conceptual model IVIVE.
IVIVE for calculating internal hazard characterisations
Translate the (external) PoD of the index substance substance to an internal hazard characterisation for the human target system/compartment.
If the RPFs are obtained are obtained using mol-based specification of the doses, then convert the mol-based RPFs to mass-based RPFs. I.e.,
Derive internal hazard characterisations for the human target system for the other substances by multiplying the RPF obtained from dose-response modelling with the hazard characterisation of the index substance. I.e.,
IVIVE for calculating external hazard characterisations
Translate the PoD of the index substance to an external human hazard characterisation (dietary/oral exposure route).
Derive an internal hazard characterisation for the index substance, with an target organ/compartment representative for the response of the dose-response model.
If the RPFs are obtained are obtained using mol-based specification of the doses, then convert the mol-based RPFs to mass-based RPFs.
Derive internal hazard characterisations for the human target system for the other substances by multiplying the RPF obtained from dose-response modelling with the hazard characterisation of the index substance.
Convert the internal hazard characterisations of the other substance to external hazard characterisations for the dietary/oral exposure route using.