Bisphenol model
Reference: Karrer et al. 2019: Karrer et al. (2019)
‘Structural analogs such as the bisphenols S, F, and AF (BPS, BPF, BPAF) are used to replace the endocrine disrupting chemical bisphenol A (BPA), but they exert estrogenic effects in the same order of magnitude. In order to investigate the consequences of BPA restrictions, we assessed the cumulative risk from BPA, BPS, BPF, and BPAF in Europe before and after the first BPA restrictions in 2011. We modelled external exposures from food, personal care products (PCPs), thermal paper, and dust, using the models MCRA and PACEM for food and PCPs, respectively. We calculated internal concentrations of unconjugated BPs with substance-specific PBPK models and cumulated concentrations by taking into account relative estrogenic potencies. Average cumulative exposure to unconjugated BPs was 3.8 and 2.1 ng/kg bw/day before and after restrictions, respectively. The decline was mostly caused by the replacement of BPA with BPS in thermal paper. Therefore, the margins of exposure (MOEs) for estrogenic effects were mostly higher after the restrictions. However, in high uncertainty percentiles the MOEs were partly lower than before (e.g. the MOEs for the uncertainty P97.5 of the variability P99 were 2.6 and 1.9 before and after restrictions, respectively), which shows the higher uncertainty around exposures for substitutes compared to BPA.’
Abstract: Linking probabilistic exposure and pharmacokinetic modelling to assess the cumulative risk from the bisphenols BPA, BPS, BPF, and BPAF for Europeans. Authors: Cecile Karrer, Waldo de Boer, Christiaan Delmaar, Yaping Cai, Amélie Crépet, Konrad Hungerbühler, Natalie von Goetz
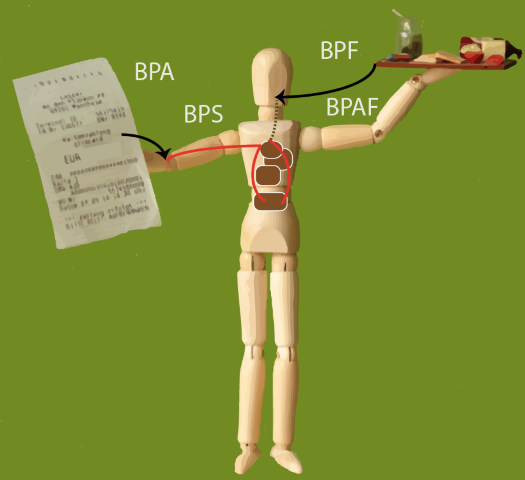
Figure 82 Graphical abstract ‘Linking probabilistic exposure and pharmacokinetic modelling to assess the cumulative risk from the bisphenols BPA, BPS, BPF, and BPAF for Europeans.’